A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC

A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC

A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC

A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. - Abstract - Europe PMC

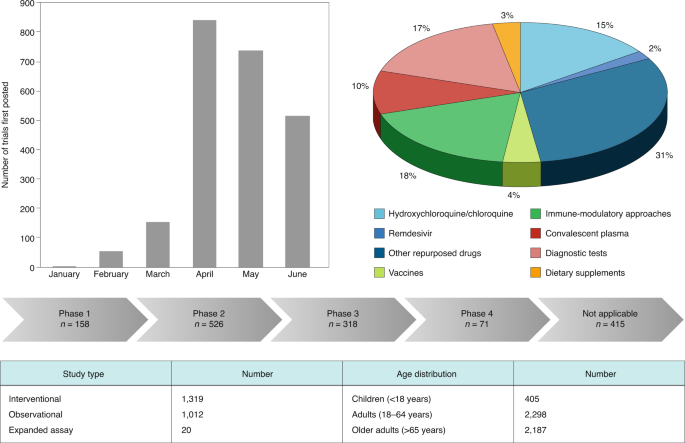
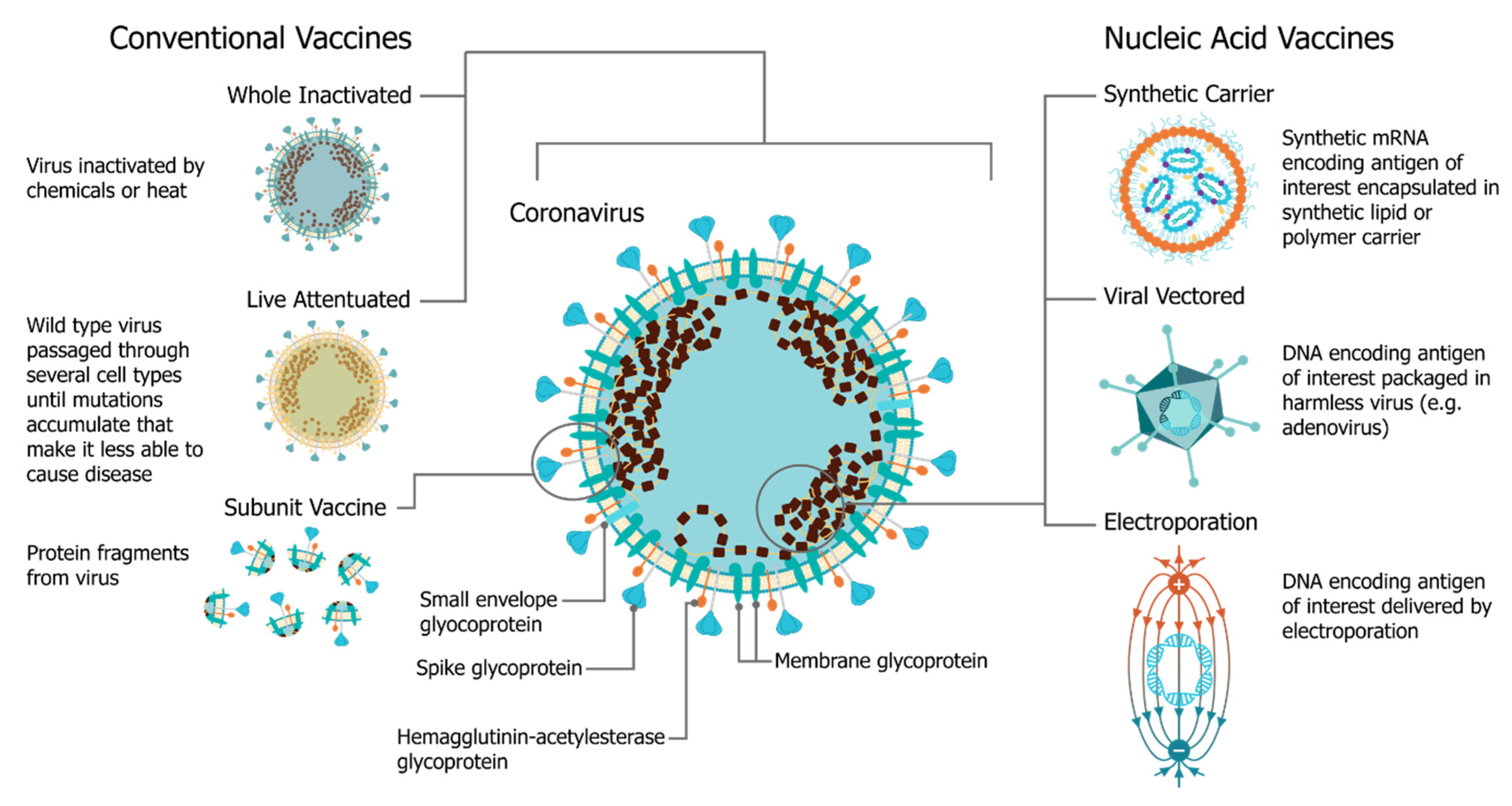
Vaccines for COVID‐19 - Tregoning - 2020 - Clinical & Experimental Immunology - Wiley Online Library
Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses
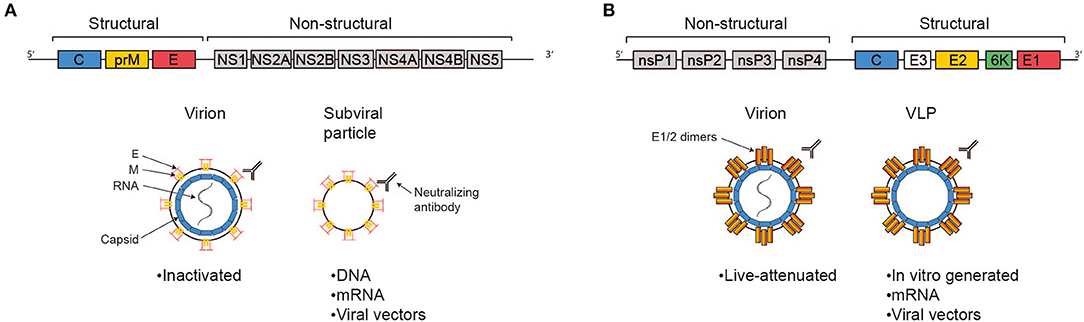
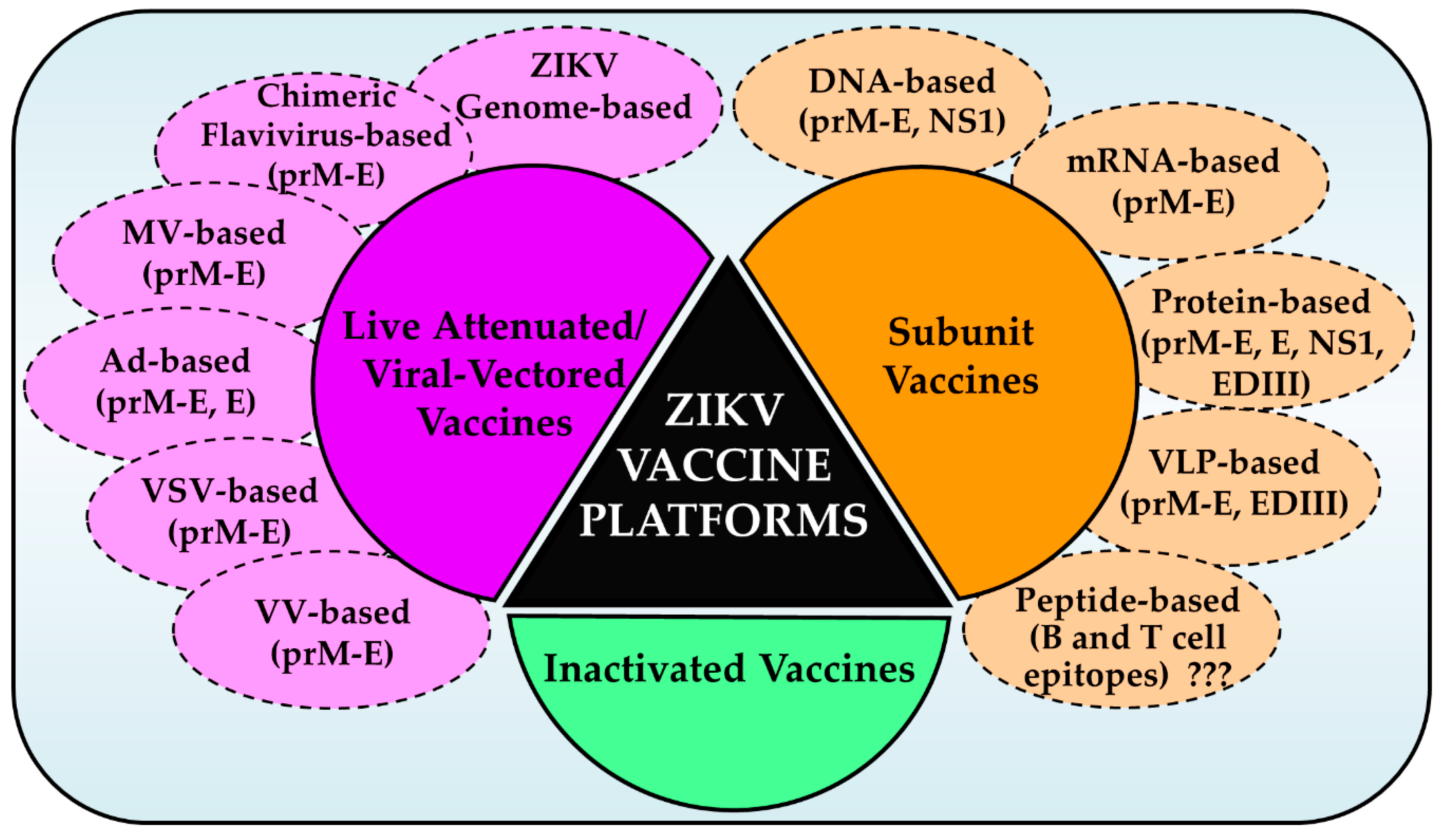
Frontiers | Current Efforts in the Development of Vaccines for the Prevention of Zika and Chikungunya Virus Infections | Immunology
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-C